Metal pickling by inorganic acids 5 – hydrofluoric acid “HF”
I would like to give a brief overview of those inorganic acids, which are traditionally used in pickling applications.
Hydrofluoric acid:
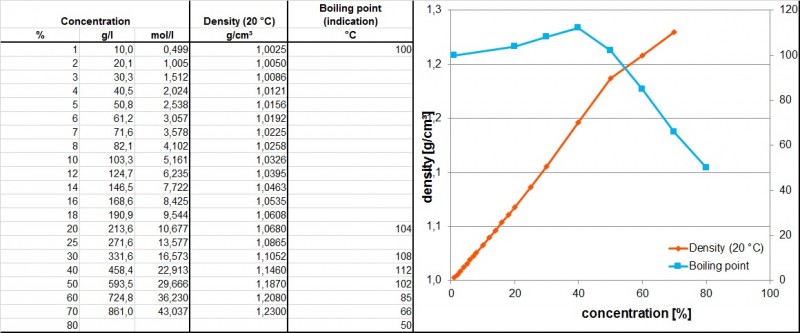
Pure hydrofluoric acid has a molar mass of 20.0 g/mol, a boiling point of 20 ° C (Riedel Inorganic Chemistry 5th edition) and is completely miscible with water. It is a colorless, corrosive, volatile liquid with a pungent odor. The usual available concentrations are in the range of 50 to 70 % (m/m). They tend to heavy smoke formation at these concentrations and ambient conditions. Hydrofluoric acid is very hygroscopic, has an azeotropic point at 38.2 %(w/w) with a boiling point of 112.2 °C and a density of 1.14 t/m³.
The table shows the density and concentration as well as the related boiling points of hydrofluoric acid. The numbers are taken from the book Beizen von Metallen, Dr. Ralf Rituper, Eugen G. Leuze Verlag Anhang 8a und 8b
Hydrofluoric acid behaves as a monovalent acid wherein forming a stable ion-pair bond between the H3O + and F-ions.
HF + H2O <-> [H3O+F-]<->H3O+ + F-
This behavior leads to a reduction of the free ions and so the aqueous HF solutions reacts as weaker acid than, for example, hydrochloric acid. With the exception of gold and platinum hydrofluoric acid dissolves most metals and metal oxides. There is a high temperature and concentration dependence for the dissolution of materials. Frequently the presence of an oxidizer (peroxide, nitrogen oxides, chlorine, sulfuric acid, …) is required.
Comparable with the hydrochloric acid behavior, most of the metals are resistant to an anhydrous HF. A passivation of the surface take place and hydrogen is formed during the passivation procedure. Iron is resistant to a HF concentration of up to 70 %(w/w). Platinum metals, silver, technical carbon are resistant even to diluted hydrofluoric acid at increasing temperatures. This is valid if there is no oxidation substance present.
The most important property of hydrofluoric acid is to solve silicic acid and salts thereof. So HF is particularly suitable for pickling sand casted products. In combination with oxidizing substances HF is used for etching stainless steel, titanium, tungsten, molybdenum, niobium, zirconium, tantalum, and their alloys.
data source: Beizen von Metallen, Dr. Ralf Rituper, Eugen G. Leuze Verlag
Hydrofluoric acid is a strong contact poison and is absorbed by the skin which can occur particularly serious injuries.
See also the blog posts on other inorganic acids: hydrochloric acid; sulfuric acid; nitric acid; phosphoric acid

Leave a Reply